Skeletal Mechanobiology and Bone Cell Networks
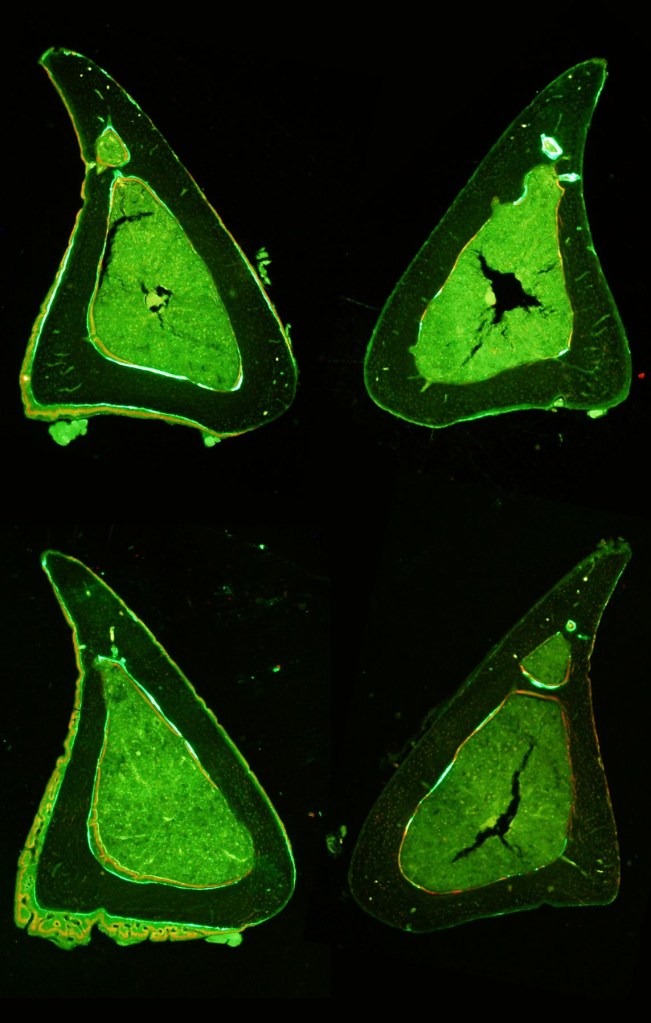
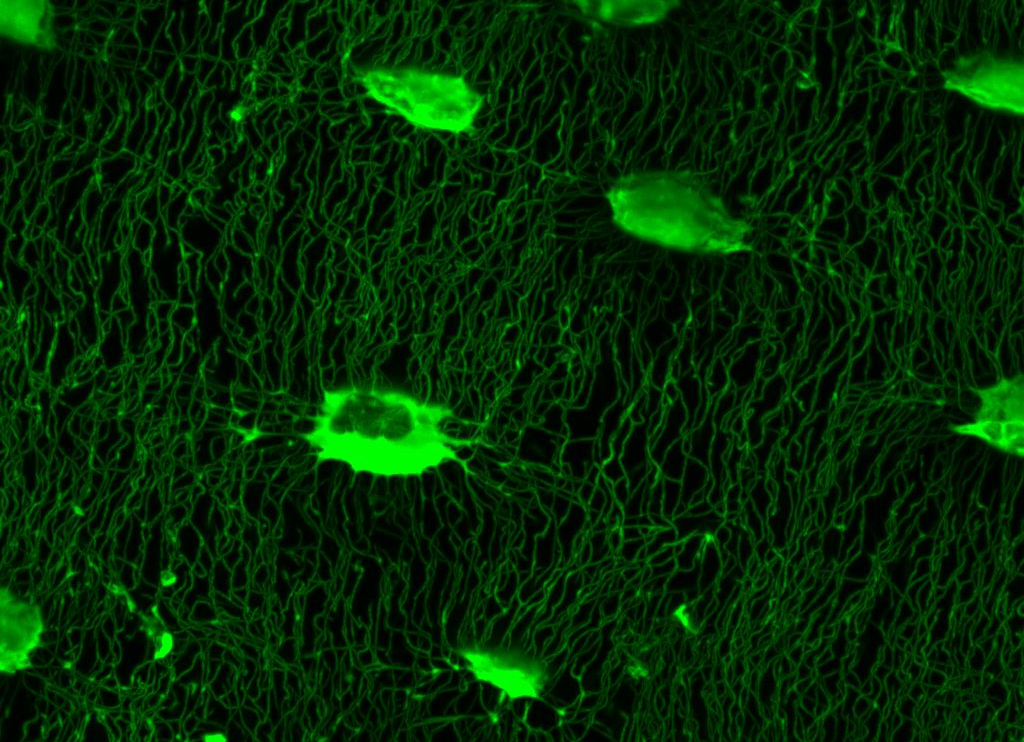
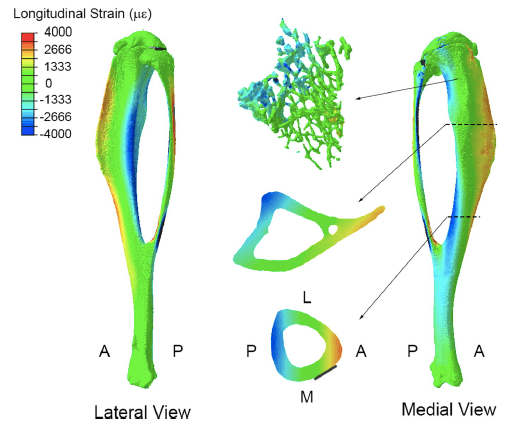
The vertebrate skeleton appears to respond to both increased physiological loads and novel loading regimes. But which bone cells are best suited to sense novel load stimuli – osteoblasts, osteocytes, or some other bone cells? Is the skeleton more sensitive to increased levels of habitual load or completely novel load events? How do the bone cells perceive these different milieu? We utilize mouse in vivo loading models and 3D confocal imaging of the osteocyte lacunar-canalicular network (LCN) to construct whole bone mechanical models to relate to cell-level mechanical stimuli induced by different types of whole-bone loading.
Just as the topology of the cellular network may influence the way that bone cells perceive local tissue strains, so too may formation of this bone cell network be influenced by differences in local strains caused by whole bone loading. To this end, we are investigating the influence of different loading regimes on characteristics of bone cell network topology as a way of determining whether characteristics of this network may be indicative of whole bone loading. Such relationships can be used in comparative models to estimate whole bone loading in fossil and extant taxa as well as in patients afflicted with skeletal wasting diseases to investigate possible causes for malfunction of mechanosensitive mechanisms in the elderly.
Contact
Purdue Musculoskeletal Biology and Mechanics Lab
Purdue University College of Veterinary Medicine
625 Harrison Street
West Lafayette, IN 47907
Phone: 765.494.0898
Fax: 765.494.0781
Email: rmain@purdue.edu